

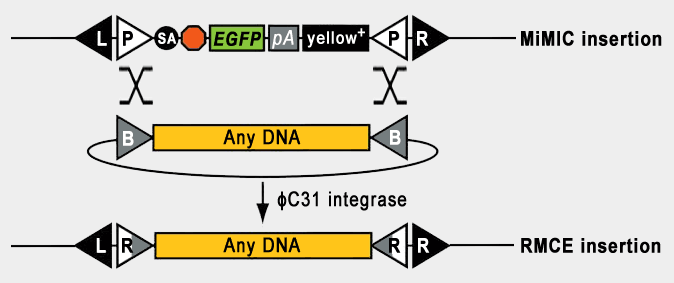
 |
| Mi{MIC} (Venken et al., 2011) is a highly versatile transposable element based on Minos (Franz et al., 1992). Minos integrates randomly into the genome (Metaxakis et al. 2005). Hence, about 30% of insertions are in introns and therefore ideal for protein trapping. Between the 255 nt Minos inverted repeats (black L and R triangles in this figure), we integrated a pair of attP cassettes (white P triangles) for Recombination Mediated Cassette Exchange (RMCE) using the ΦC31 integrase (Bateman et al., 2006). This allows the stable integration of any DNA fragment in vivo. The attP sites within the MiMIC insertion recombine with attB sites (gray B triangles) within the designed plasmid, thereby integrating the desired cassette at the location of the MiMIC in the genome. |
| We have deposited to the DGRC the following constructs for RMCE: correction (no insert), gene trap (incorporates a splice acceptor site and effectors/markers), and protein trap (incorporates splice acceptor and splice donor sites and tags/markers). |
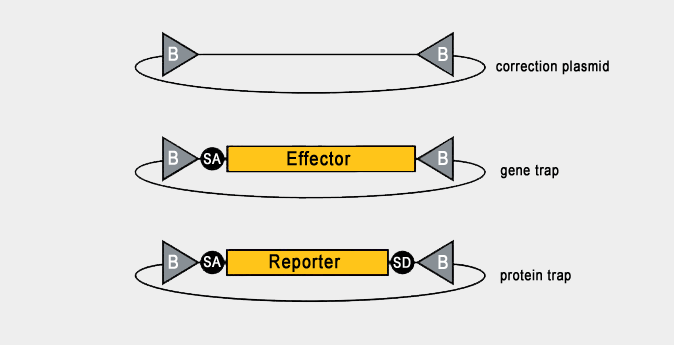 |
| Hence, depending on the position of the starting Mi{MIC} insertion within the genome, different RMCE plasmids can be selected to achieve different ends. For example, if you desire to tag your gene of interest with a fluorescent reporter, you require a Mi{MIC} insertion within an intron, and a plasmid containing both splice acceptor and splice donor sites flanking the reporter sequence. Subsequently, the fluorescent tag is incorporated into the resultant protein in between coding exons. |
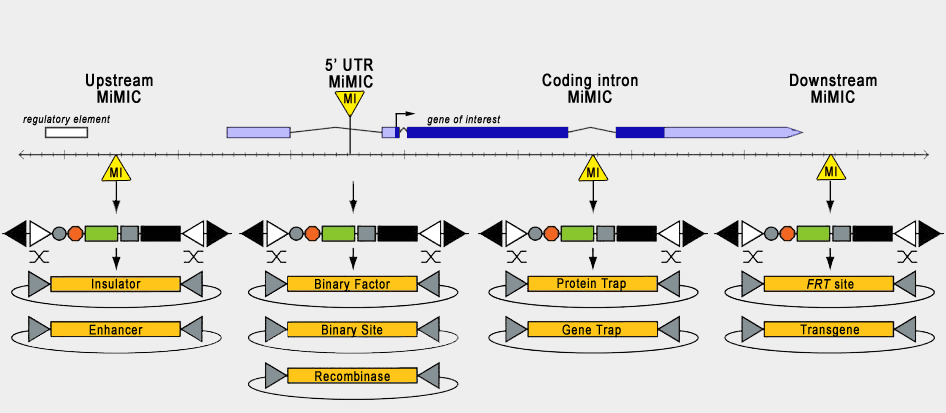
|
| We have generated a method to introduce the reporter construct by genetic crosses rather than injection (see Nagarkar-Jaiswal et al. (2015) A genetic toolkit for tagging intronic MiMIC containing genes. eLife 4:e08469.) Details of the procedure and stocks to order are outlined on the Crossing Page. |
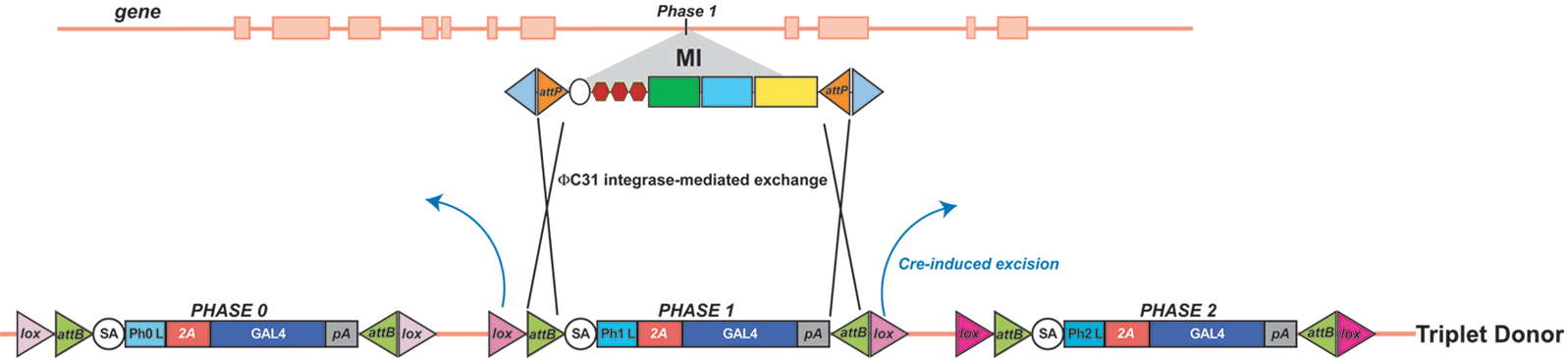
| We have recently converted strategies from using the GFSTF reporter for RMCE to the Trojan-GAL4 method of (Diao et al., 2015). Please see the publication and the below diagram (an example for a Phase 1 MiMIC insertion) for an outline of the strategy. All stocks are available from Bellen Lab until deposited into BDSC.
Reference: Diao F, Ironfield H, Luan H, Diao F, Shropshire WC, Ewer J, Marr E, Potter CJ, Landgraf M, White BH (2015) Plug-and-play genetic access to drosophila cell types using exchangeable exon cassettes. Cell Reports 10:1410-1421.[Abstract | PDF] |
 |